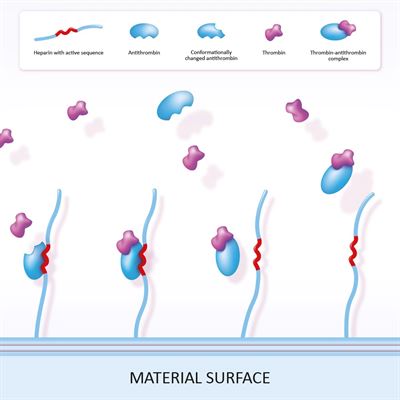
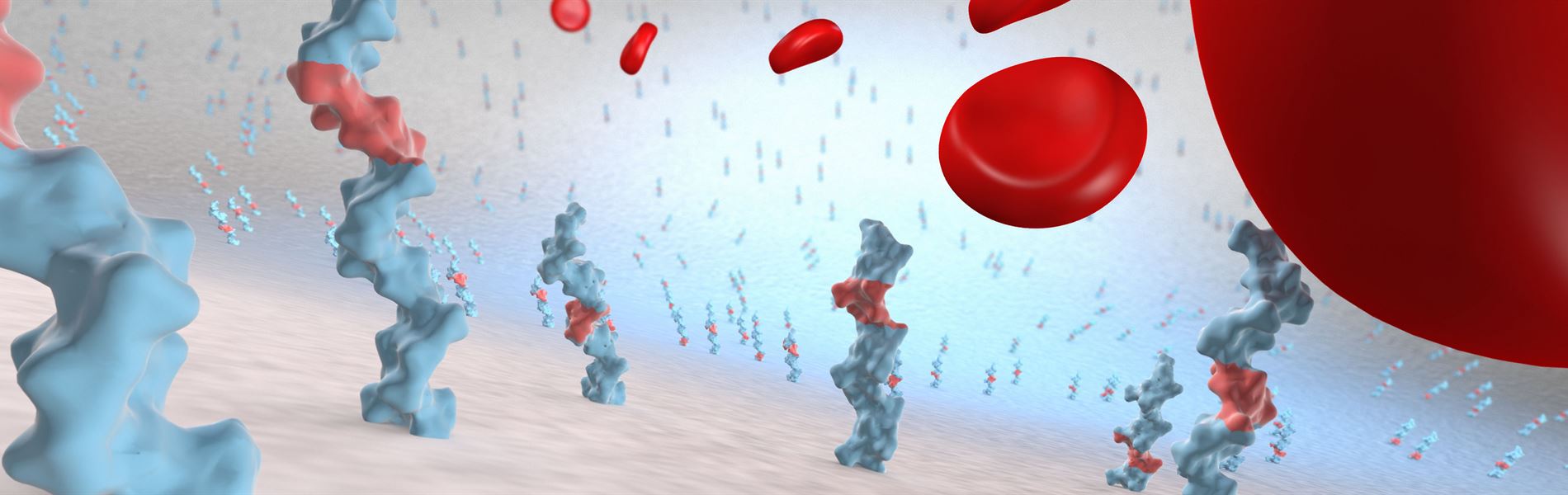
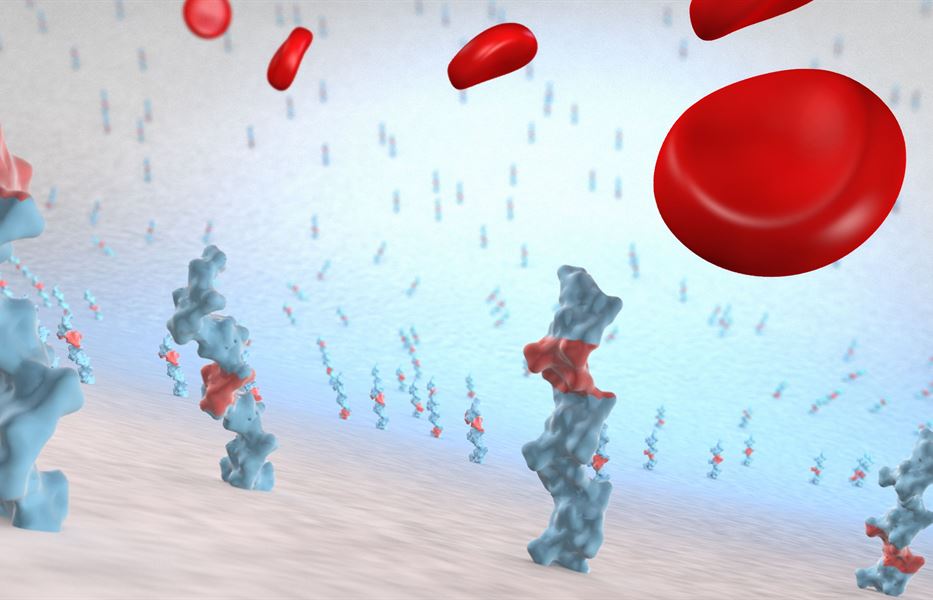
The CARMEDA® BioActive Surface is a clinically proven and lasting thromboresistant heparin coating that actively prevents platelet adhesion and thrombus formation on medical device surfaces.
The following features and benefits of the CARMEDA® BioActive Surface have been shown in published clinical studies and scientific papers:
Thromboresistant - By suppressing the coagulation mechanism the CARMEDA® BioActive Surface reduces or eliminates thrombotic complications related to the exposure of blood to artificial materials (1, 2). The CARMEDA® BioActive Surface has been shown to effectively neutralize activated coagulation factors such as thrombin (3, 4) and FXa (5). Studies have also revealed that the initiation mechanism of the coagulation system is suppressed by the bound heparin (6, 7). On ePTFE vascular grafts the Carmeda heparin technology has been shown to provide a significantly improved thromboresistance (8, 9). The CARMEDA® BioActive Surface has in certain applications reduced the risk of thrombosis-related complications to a degree that allowed decreased systemic anticoagulation (10, 11).
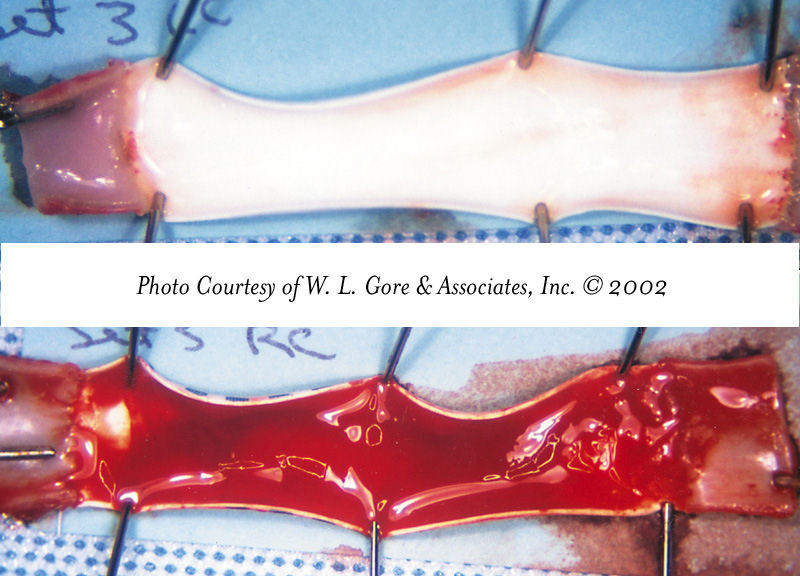
Luminal surfaces of artificial blood vessels after implantation in a challenging carotid shunt canine model. The upper image shows a material featuring the thromboresistant CARMEDA® BioActive Surface, which remains free of thrombus. The lower image shows a conventional artificial material, which is covered with thrombus.
Platelet compatible - The CARMEDA® BioActive Surface causes minimal activation and adhesion of platelets as demonstrated by in vitro (12, 13, 14), in vivo (13, 15) and clinical studies (16, 17).
Decreased inflammatory response - Several preclinical studies have shown near-elimination of complement activation in blood exposed to the CARMEDA® BioActive Surface (18, 19). The CARMEDA® BioActive Surface has also been demonstrated to effectively prevent complement-dependent as well as complement-independent activation of cells such as leukocytes and platelets (20) along with inhibition of increases in chemokines and growth factors (21). Clinical studies have confirmed that medical devices with the CARMEDA® BioActive Surface cause less complement activation than uncoated devices (22, 16).
Reduced infection rates - Clinical studies have indicated a reduced infection rate in patients treated with central venous catheters with CARMEDA® BioActive Surface compared to uncoated catheters (23, 24). Hypothetically, due to the thromboresistant properties of the CARMEDA® BioActive Surface, the catheter surface is less likely to be fouled by clot-related material, making it less prone to support microbial growth.
Reduced neointimal hyperplasia - Several studies in animal models have shown that vascular grafts coated with the Carmeda heparin technology delay and reduce anastomotic intimal hyperplasia, both short- and long term at 2 years (9, 13, 15, 25).
Lasting performance - The CARMEDA® BioActive Surface is a long-term, highly robust thromboresistant coating able to withstand all expected fluid flow conditions and many mechanical challenges. For example, the coating has been shown to remain bound and functional on explanted devices after months or even years of blood flow contact (26, 27, 28). It also withstands, e.g. the mechanical challenge of balloon expansion of coronary stents in stenosed arteries (13, 29).
Improved clinical outcome - Numerous studies have shown improved clinical outcome for devices featuring the CARMEDA® BioActive Surface. Direct comparison of uncoated and coated ventricular assist devices (VADs) have shown that those with the CARMEDA® BioActive Surface significantly reduce the replacement rate of the pumps, mainly due to decreased thrombus deposition and thromboembolic complications (30). The clinical benefit of vascular grafts has similarly been investigated in lower extremity revascularization, where ePTFE grafts coated with the Carmeda heparin technology have superior primary patency rates in both above-knee and below-knee grafts when compared to non-heparin coated grafts (31, 32). These grafts configured for pediatric shunts have also been shown to reduce mortality in neonates and infants receiving systemic-to-pulmonary shunts (33). Compared with non-heparin coated ePTFE grafts they have had fewer shunt occlusions/thromboses and desaturation or arrest events, resulting in significantly lower shunt-related as well as overall 30-day mortality. Another example is coronary stents, where stents coated with the CARMEDA® BioActive Surface reduced subacute stent thrombosis in coronary interventions compared to bare metal stents (34). An extensive number of clinical references categorized into type of product/application can be found in the CARMEDA® BioActive Surface reference list (see link below).
Superiority compared to other commercial heparin coatings - Central to the design of a functional heparin surface is to retain the anticoagulant activity of heparin, i.e. by preserving its ability to bind antithrombin and catalyze the inhibition of coagulation factors. Of the various approaches and methods used to bond heparin permanently, very few achieve this prerequisite of preserving antithrombin binding. It is thus important to recognize that not all heparin coatings are equivalent (35). Of commercialized non-eluting heparin coating technologies developed for use in the medical device industry, few technologies except the CARMEDA® BioActive Surface have publications showing biochemical evidence of surface heparin functionality (36).
Follow the link for references: Features & Benefits references
Or download a pdf with text and references: Features & Benefits references
The CARMEDA® BioActive Surface has an impressive pre-clinical and clinical track record, and undoubtedly an extensive publication history describing both basic biochemical mechanisms and clinical applications. For a full reference list download the CARMEDA® BioActive Surface Reference List

CBAS® Heparin Surface
The CARMEDA® BioActive Surface is marketed under the trademark CBAS® Heparin Surface for GORE® Vascular Devices.
Coating Technology
Proprietary end-point attached heparin with retained bioactivity.